- Call us: 01725062700
- Email: info@sanctusglobal.com
Generic Pharma Company
Generic Pharma Company Franchise - In India, the pharma industry is developing and manufacturing rapidly in recent years. Our company is the largest manufacturer and advertiser of pharmaceutical products. Sanctus Global is distributing traditional medicine medicines through manufacturing technology covering a significant portion of the global health market. Along these lines, assuming that you are looking for a top Generic Pharma Company Franchise in India, we will do our best to help you. Till now, India is exporting 20 percent of the world's medicines.
This figure is not on the top list. The situation of pharmaceutical drugs in our country is continuously increasing. Apart from this, it is also picking the top Generic Medicines PCD Franchise companies in India. Before the year 2025, it is typical for the pharmaceutical business in India to grow by 15-20%. This means that the Indian pharmaceutical industry will increase to $100 billion, which is really a lot.
Presently people are investing resources in the pharma industry in light of their growth perception. There are a ton of pharma franchise companies out there, yet it is exceptionally important to have the best resources you can to get the best results. In this way, Sanctus Global is working every day to become the most acclaimed generic medicine franchise company in India. To interface with us for Franchisee services, you can call us at +91 9914562999. Apart from this, you can also mail us at SALES@SANTUSGLOBAL.COM.
We manufacture a wide range of oral solid dosage forms. Here is an illustrative list of the products we currently manufacture. In addition, we can manufacture all nutraceuticals (Oral solids) and any other non-β-lactam formulation.
GASTRO INTESTINAL DRUGS
Sr. No. |
Product Name |
Composition |
Remarks |
|---|---|---|---|
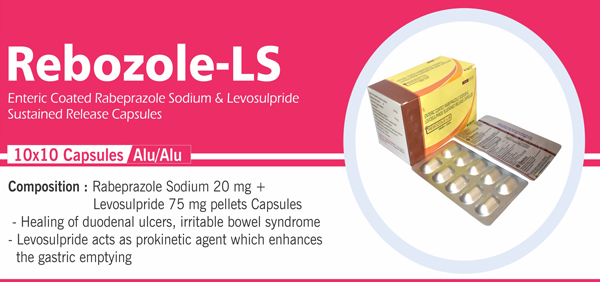
01 |
Rabeprazole Sodium Gastro – Resistant Tablets IP 20 mg |
Each Enteric Coated Tablet Contains: Rabeprazole Sodium IP 20 mg Colours : Approved colours |
|
02 |
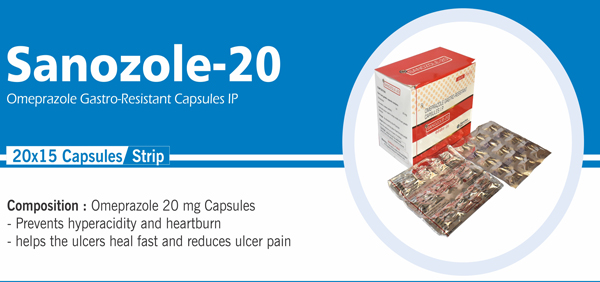
Omeprazole Gastro Resistant |
Each hard gelatin capsule contains: Omeprazole IP 20 mg |
|
03 |
Pantoprazole Gastro – Resistant |
Each enteric coated tablet contains: Pantoprazole Sodium Sesquihydrate IP eq. to Pantoprazole 20 mg Excipients q.s. |
|
04 |
Pantoprazole Gastro – Resistant |
Each enteric coated tablet contains: Pantoprazole Sodium Sesquihydrate IP |
|
05 |
Pantoprazole Sodium and |
Each enteric coated tablet contains: Pantoprazole Sodium Sesquihydrate IP eq. |
|
06 |
Pantoprazole Sodium and |
Each enteric coated tablet contains: Pantoprazole Sodium Sesquihydrate IP |
|
07 |
Pantoprazole Sodium and |
Each hard gelatin capsule contains: Pantoprazole Sodium Sesquihydrate IP |
|
08 |
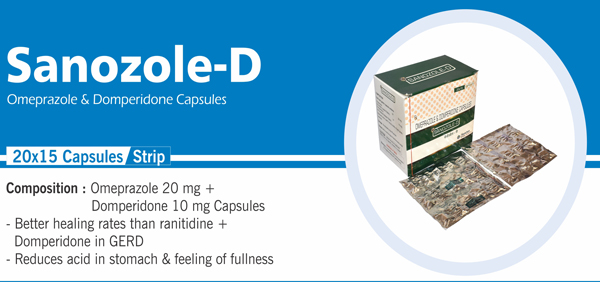
Rabeprazole and Domperidone |
Each enteric coated tablet contains: Rabeprazole Sodium IP 20 mg |
|
09 |
Esomeprazole Magnesium and |
Each hard gelatin capsule contains: Esomeprazole Magnesium Trihydrate IP eq. to Esomeprazole 40 mg |
|
10 |
Esomeprazole Magnesium and |
Each hard gelatin capsule contains: Esomeprazole Magnesium Trihydrate IP eq. to Esomeprazole 20 mg |
|
11 |
Esomeprazole Gastro Resistant |
Each enteric coated tablet contains: Esomeprazole Magnesium Trihydrate IP |
|
12 |
Rabeprazole (EC) and |
Each hard gelatin capsule contains: Rabeprazole Sodium 20 mg |
|
ANTI-DIABETES DRUGS
Sr. No. |
Product Name |
Composition |
Remarks |
|---|---|---|---|
13 |
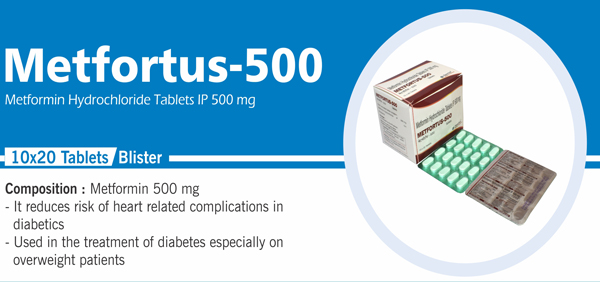
Metformin Hydrochloride Tablets |
Each uncoated tablet contains: Metformin Hydrochloride IP 500 mg |
|
14 |
Glimepiride and Metformin |
Each uncoated bilayered tablet contains: Glimepiride IP 1 mg Metformin Hydrochloride IP 1000 mg |
|
15 |
Glimepiride and Metformin |
Each uncoated bilayered tablet contains: Glimepiride IP 2 mg |
|
16 |
Metformin Hydrochloride |
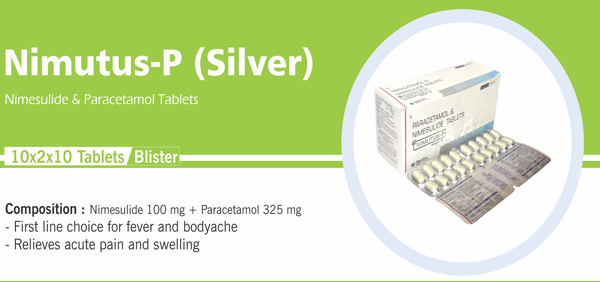
Each uncoated Prolonged- release tablet contains: |
|
17 |
Glimepiride and Metformin |
Each uncoated tablet contains : Glimepiride IP 2 mg |
|
18 |
Gliclazide and Metformin |
Each uncoated tablet contains : Gliclazide IP 80 mg |
|
19 |
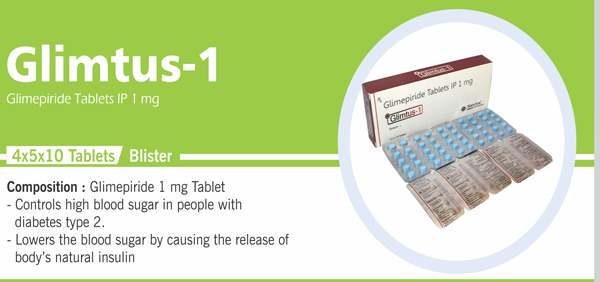
Glimepiride Tablets IP 1 mg |
Each uncoated tablet contains: Glimepiride IP 1 mg |
|
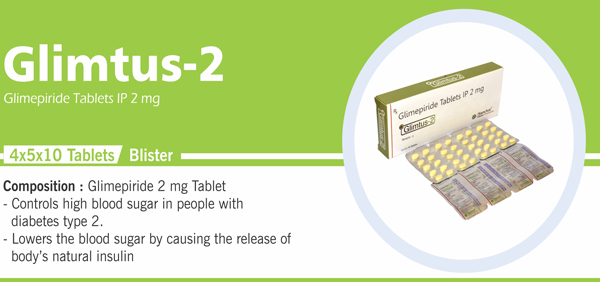
20 |
Glimepiride Tablets IP 2 mg |
Each uncoated tablet contains: Glimepiride IP 2 mg Colours: Approved Colours |
|
21 |
Glimepiride and Metformin |
Each uncoated bilayered tablet contains: Glimepiride IP 1 mg Metformin Hydrochloride IP 500 mg |
|
22 |
Glimepiride and Metformin |
Each uncoated bilayered tablet contains: Glimepiride IP 2 mg Metformin Hydrochloride IP 500 mg |
|
23 |
Gliclazide Tablets IP 80 mg |
Each uncoated tablet contains: Gliclazide IP 80 mg |
|
24 |
Glimepiride. Pioglitazone and |
Each uncoated bilayered tablet contains: Glimepiride IP 2 mg |
|
25 |
Glimepiride. Pioglitazone and |
Each uncoated bilayered tablet contains: Glimepiride IP 1 mg |
|
ANTI-HYPERTENSIVE DRUGS
Sr. No. |
Product Name |
Composition |
Remarks |
|---|---|---|---|
|
|||
26 |
Losartan Potassium and |
Each film coated tablet contains: Losartan Potassium IP 50 mg Hydrochlorothiazide IP 12.5mg Colours : Approved colours |
|
27 |
Losartan Potassium Tablets |
Each film coated tablet contains : Losartan Potassium IP 25mg |
|
28 |
Losartan Potassium Tablets |
Each film coated tablet contains : Losartan Potassium IP 50mg |
|
29 |
Losartan Potassium and |
Each film coated tablet contains: Losartan Potassium IP 50 mg |
|
30 |
Metoprolol Succinate Extended release Tablets IP |
Each film coated extended release tablet contains: |
|
31 |
Metoprolol Succinate Extended release Tablets IP |
Each film coated extended release tablet contains: |
|
32 |
Prazosin Hydrochloride |
Each film coated extended release tablet contains: |
|
33 |
Prazosin Hydrochloride |
Each film coated extended release tablet contains: |
|
34 |
Telmisartan Tablets IP 40 mg |
Each uncoated tablet contains: Telmisartan IP 40 mg |
|
35 |
Telmisartan Tablets IP 80 mg |
Each uncoated tablet contains: Telmisartan IP 80 mg |
|
36 |
Telmisartan and |
Each uncoated tablet contains : Telmisartan IP 80 mg |
|
37 |
Telmisartan and |
Each uncoated tablet contains : Telmisartan IP 40 mg |
|
38 |
Telmisartan and Amlodipine |
Each uncoated tablet contains : Telmisartan IP 80 mg Amlodipine Besylate IP Equivalent to Amlodipine 5 mg Colours: Approved Colours |
|
39 |
Propranolol Hydrochloride |
Each uncoated tablet contains: Propranolol Hydrochloride IP 40 mg |
|
40 |
Ramipril Tablets IP 2.5 mg |
Each uncoated tablet contains: Ramipril IP 2.5 mg |
|
41 |
Ramipril Tablets IP 5 mg |
Each uncoated tablet contains: Ramipril IP 5 mg |
|
42 |
Ramipril Tablets IP 10 mg |
Each uncoated tablet contains: Ramipril IP 10 mg |
|
43 |
Olmesartan Medoxomil Tablets |
Each film coated tablet contains: Olmesartan Medoxomil 20 mg Colours: Approved Colours |
|
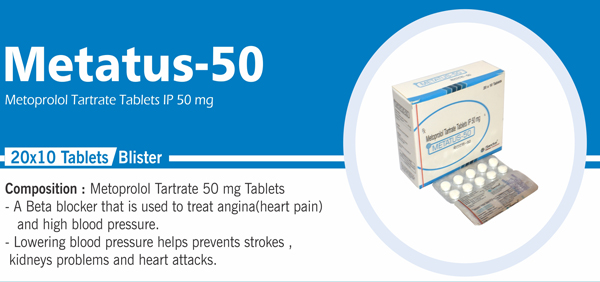
44 |
Metoprolol Tartrate |
Each uncoated tablet contains: Metoprolol Tartrate IP 25 mg |
|
45 |
Metoprolol Tartrate |
Each uncoated tablet contains: Metoprolol Tartrate IP 50 mg |
|
46 |
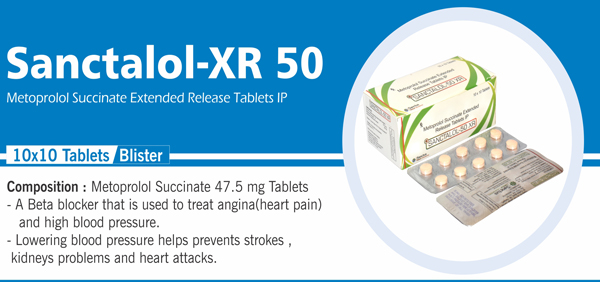
Metoprolol Succinate ER and |
Each film coated bilayered tablet contains: Metoprolol Succinate IP 23.75 mg Equivalent to Metoprolol Tartrate 25 mg (As extended release) |
|
47 |
Metoprolol Succinate ER and |
Each film coated bilayered tablet contains: Metoprolol Succinate IP 47.50 mg Equivalent to Metoprolol Tartrate 50 mg (As extended release) |
|
48 |
Cilnidipine Tablets 5 mg |
Each film coated tablet contains: Cilnidipine 5 mg |
|
49 |
Cilnidipine Tablets 10 mg |
Each film coated tablet contains: Cilnidipine 10 mg |
|
50 |
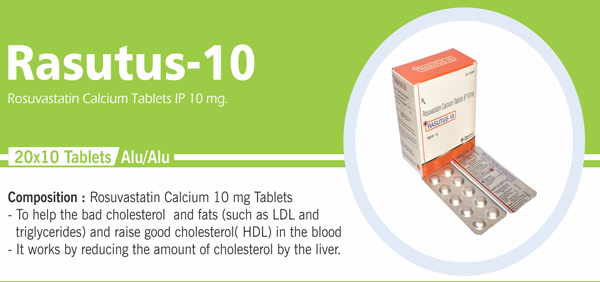
Rosuvastatin Calcium Tablets IP |
Each film coated tablet contains: Rosuvastatin Calcium IP |
|
51 |
Rosuvastatin Calcium Tablets IP |
Each film coated tablet contains: Rosuvastatin Calcium IP Equivalent to Rosuvastatin 10 mg Excipients q.s Colours: Approved Colours |
|
52 |
Rosuvastatin Calcium Tablets IP |
Each film coated tablet contains: Rosuvastatin Calcium IP Equivalent to Rosuvastatin 20 mg Excipients q.s Colours: Approved Colours |
|
CARDIO VASCULAR DRUGS
Sr. No. |
Product Name |
Composition |
Remarks |
|---|---|---|---|
53 |
Clopidogrel Tablets IP 75 mg |
Each film coated tablet contains : Clopidogrel Bisulphate IP |
|
54 |
Atorvastatin Calcium Tablets IP |
Each film coated tablet contains: Atorvastatin Calcium IP Equivalent to Atorvastatin 10 mg Colours : Approved colours |
|
55 |
Atorvastatin Calcium Tablets IP |
Each film coated tablet contains : Atorvastatin Calcium IP |
|
ANTI-BIOTICS
Sr. No. |
Product Name |
Composition |
Remarks |
|---|---|---|---|
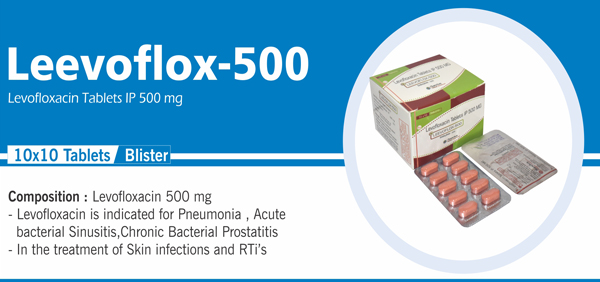
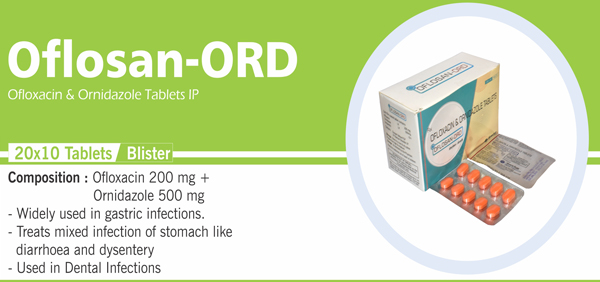
56 |
Ciprofloxacin and Ornidazole |
Each film coated tablet contains: Ciprofloxacin hydrochloride IP Equivalent to Ciprofloxacin 500 mg Ornidazole IP 500 mg |
|
57 |
Ofloxacin and Ornidazole |
Each Film Coated Tablet Contains : Ofloxacin IP 200 mg |
|
58 |
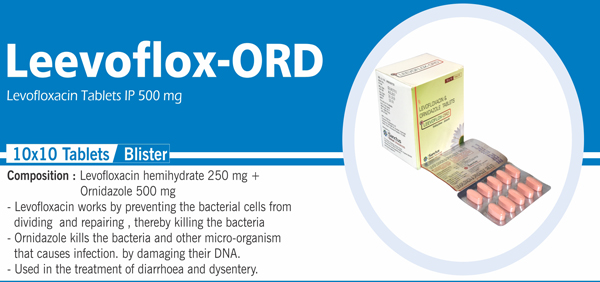
Levofloxacin Hemihydrate and |
Each film coated tablet contains: Levofloxacin Hemihydrate IP |
|
59 |
Ofloxacin Tablets IP 200 mg |
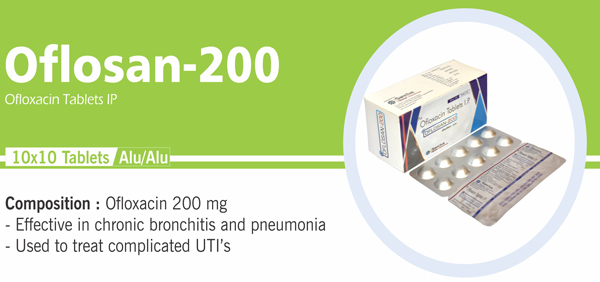
Each film coated tablet contains: Ofloxacin IP 200 mg |
|
60 |
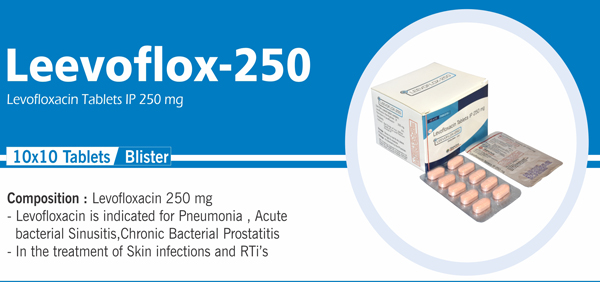
Levofloxacin Tablets IP 250 mg |
Each film coated tablet contains: Levofloxacin Hemihydrate IP |
|
61 |
Levofloxacin Tablets IP 500 mg |
Each film coated tablet contains: Levofloxacin Hemihydrate IP |
|
62 |
Levofloxacin Tablets IP 750 mg |
Each film coated tablet contains: Levofloxacin Hemihydrate IP |
|
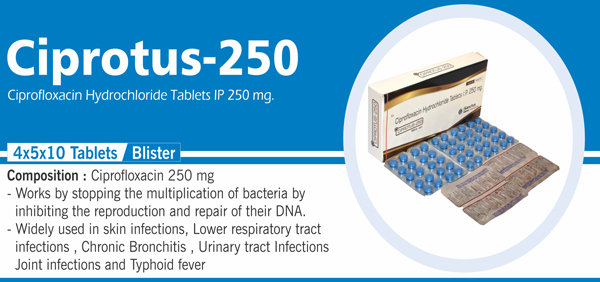
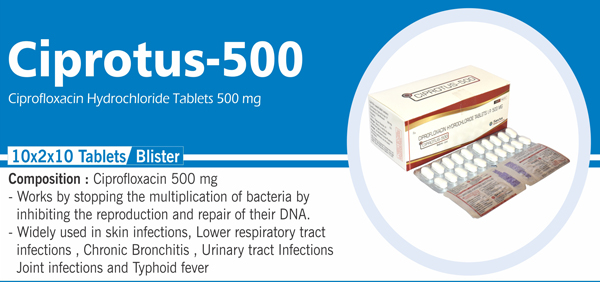
63 |
Ciprofloxacin Hydrochloride IP |
Each film coated tablet contains: Ciprofloxacin Hydrochloride IP eq. to Ciprofloxacin 500 mg Colours : Approved colours |
|
64 |
Ciprofloxacin Hydrochloride IP |
Each film coated tablet contains: Ciprofloxacin Hydrochloride IP |
|
65 |
Ciprofloxacin Hydrochloride and Tinidazole Tablets |
Each film coated tablet contains: Ciprofloxacin Hydrochloride IP Equivalent to Ciprofloxacin 500 mg Tinidazole IP 600 mg Colours : Approved colours |
|
66 |
Azithromycin Tablets IP 500 mg |
Each film coated tablet contains: Azithromycin dihydrate IP |
|
67 |
Azithromycin Tablets IP 250 mg |
Each film coated tablet contains: Azithromycin dihydrate IP Equivalent to Azithromycin 250 mg Excipients q.s. |
|
ANALGESIC & ANTIPYRETICS
Sr. No. |
Product Name |
Composition |
Remarks |
|---|---|---|---|
68 |
Paracetamol Tablets IP 325 mg |
Each uncoated tablet contains: Paracetamol IP 325 mg |
|
69 |
Paracetamol Tablets IP 500 mg |
Each uncoated tablet contains: Paracetamol IP 500 mg |
|
70 |
Paracetamol Tablets IP 650 mg |
Each uncoated tablet contains: Paracetamol IP 650 mg |
|
71 |
Tramadol Hydrochloride |
Each hard gelatin capsule contains: Tramadol Hydrochloride BP 100 mg Excipients q.s. Approved colours used in empty hard gelatin capsule shells |
|
72 |
Thiocolchicoside and Etoricoxib |
Each film coated tablet contains: Thiocolchicoside IP 4 mg Etoricoxib 60 mg |
|
73 |
Lornoxicam and Paracetamol |
Each film coated tablet contains: Lornoxicam IP 4 mg Paracetamol IP 325 mg |
|
74 |
Aceclofenac and Tizanidine |
Each film coated tablet contains: Aceclofenac IP 100 mg Tizanidine Hydrochloride IP 2 mg Colours : Approved colours |
|
75 |
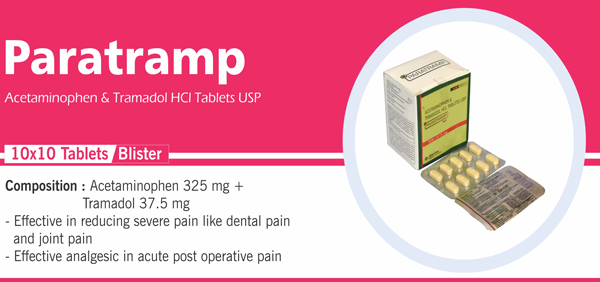
Acetaminophen and Tramadol |
Each film coated tablet contains: Acetaminophen IP 325 mg Tramadol Hydrochloride IP 37.5 mg Colour: Approved colours(s) |
|
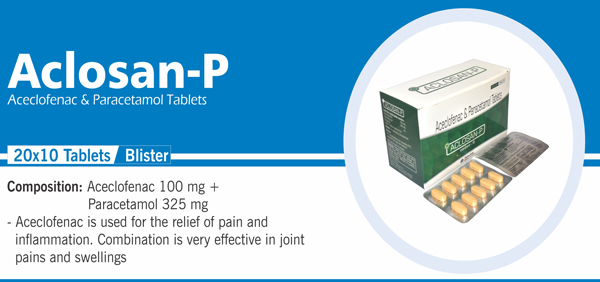
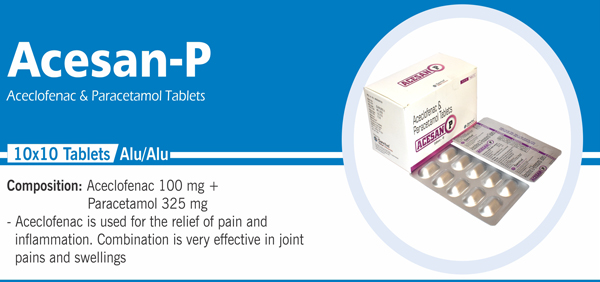
76 |
Aceclofenac and Paracetamol |
Each film coated tablet contains: Aceclofenac IP 100 mg |
|
77 |
Aceclofenac, Paracetamol and |
Each film coated tablet contains: Aceclofenac IP 100 mg |
|
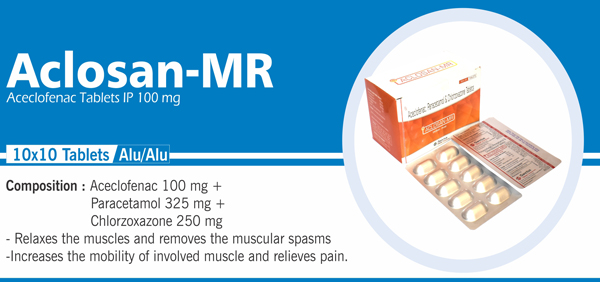
78 |
Aceclofenac , Paracetamol and |
Each film coated tablet contains: Aceclofenac IP 100 mg Paracetamol IP 325 mg Chlorzoxazone USP 250 mg Colours : Approved colours |
|
79 |
Diclofenac Potassium and |
Each uncoated tablet contains: Diclofenac Potassium BP 50 mg |
|
80 |
Aceclofenac Tablets IP 100 mg |
Each film coated tablet contains : Aceclofenac IP 100 mg |
|
81 |
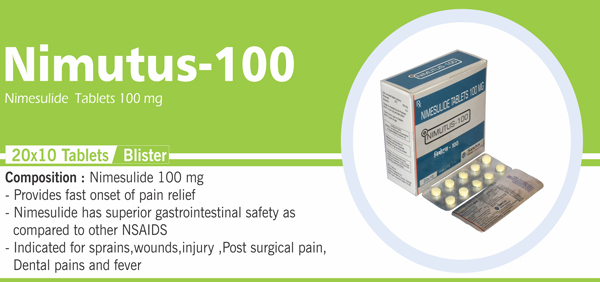
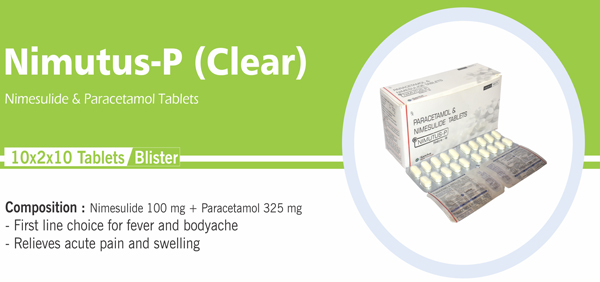
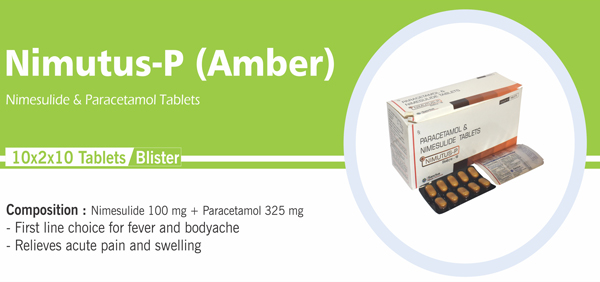
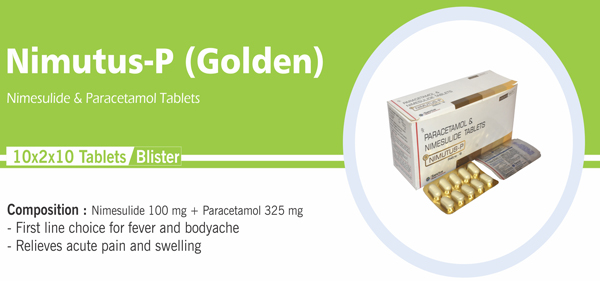
Nimesulide and Paracetamol |
Each uncoated bilayered tablet contains : Nimesulide BP 100 mg |
|
82 |
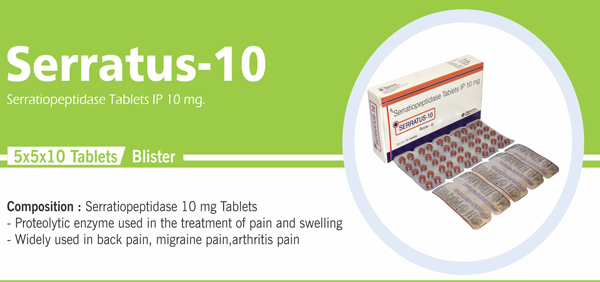
Serratiopeptidase Tablets IP 10 mg |
Each film coated tablet contains: Serratiopeptidase IP 10 mg |
|
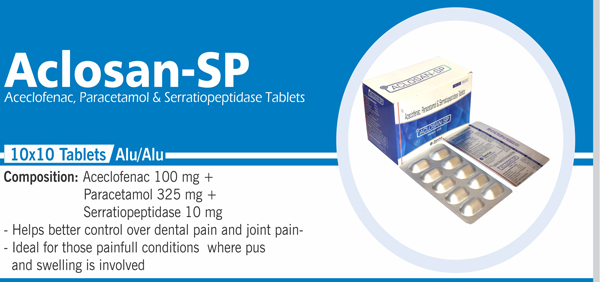
83 |
Aceclofenac , Paracetamol and |
Each film coated tablet contains: Aceclofenac IP 100 mg Paracetamol IP 325 mg |
|
84 |
Mefenamic Acid and |
Each uncoated tablet contains: Mefenamic Acid IP 250 mg |
|
NON STEROIDAL ANTI-INFLAMMATORY DRUGS (NSAID)
Sr. No. |
Product Name |
Composition |
Remarks |
|---|---|---|---|
85 |
Etodolac Extended Release |
Each film coated extended release tablet contains: |
|
86 |
Mefenamic Acid and |
Each uncoated tablet contains |
|
87 |
Diclofenac Potassium, Paracetamol and Magnesium Trisilicate Tablets |
Each uncoated tablet contains: Diclofenac Potassium BP 50 mg Paracetamol IP 325 mg Magnesium Trisilicate IP 100 mg Colour : Approved colour(s) |
|
88 |
Nimesulide mouth dissolving tablets 100 mg |
Each uncoated dispersible tablet contains: Nimesulide BP 100 mg |
|
89 |
Mefenamic Acid Tablets BP 250 mg |
Each uncoated tablet contains: Mefenamic Acid IP 250 mg |
|
90 |
Mefenamic Acid Tablets BP 500 mg |
Each uncoated tablet contains: Mefenamic Acid IP 500 mg |
|
91 |
Diclofenac Sodium Tablets |
Each film coated tablet contains: Diclofenac Sodium IP 100 mg |
|
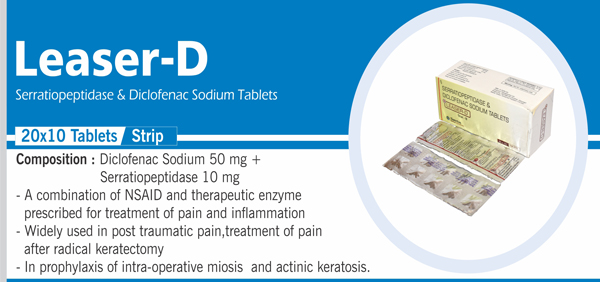
92 |
Serratiopeptidase and |
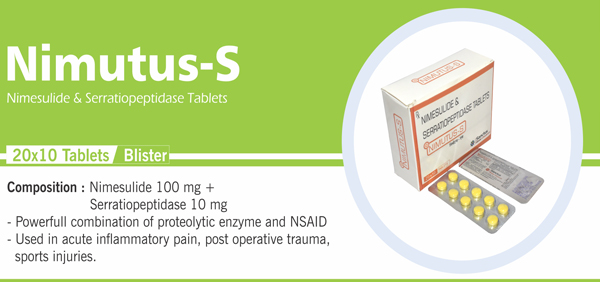
Each enteric coated tablet contains: Serratiopeptidase IP 10 mg (20,000 Serratiopeptidase units) Diclofenac Sodium IP 50 mg Colours : Approved colours |
|
93 |
Diclofenac Sodium and |
Each uncoated bilayered tablet contains: Diclofenac Sodium IP 50 mg |
|
ANTHELMINTICS DRUGS
Sr. No. |
Product Name |
Composition |
Remarks |
|---|---|---|---|
94 |
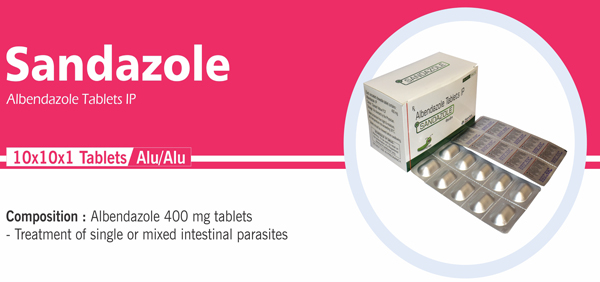
Albendazole Tablets IP |
Each uncoated chewable tablet contains: Albendazole IP 400 mg |
|
95 |
Ivermectin and Albendazole |
Each uncoated chewable tablet contains: Ivermectin IP 6 mg |
|
96 |
Ivermectin and Albendazole |
Each uncoated chewable tablet contains: Ivermectin IP 12 mg |
|
CENTRAL NERVOUS SYSTEM DRUGS
Sr. No. |
Product Name |
Composition |
Remarks |
|---|---|---|---|
97 |
Sodium Valproate Tablets IP |
Each enteric coated tablet contains : Sodium Valproate IP 200 mg |
|
98 |
Gabapentin Capsules IP |
Each hard gelatin capsules contains: Gabapentin IP 300 mg |
|
99 |
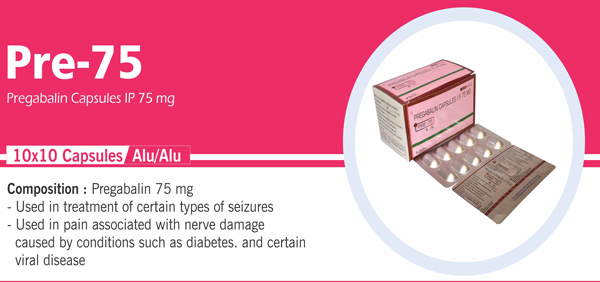
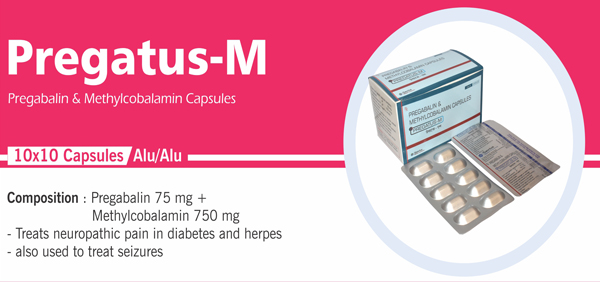
Methylcobalamin and Pregabalin |
Each hard gelatin capsule contains: Methylcobalamin JP 500 mcg |
|
100 |
Methylcobalamin and Pregabalin |
Each hard gelatin capsule contains: Methylcobalamin JP 750 mcg |
|
101 |
Methylcobalamin and Pregabalin |
Each hard gelatin capsule contains: Methylcobalamin JP 500 mcg |
|
102 |
Methylcobalamin and Pregabalin |
Each hard gelatin capsule contains: Methylcobalamin JP 750 mcg |
|
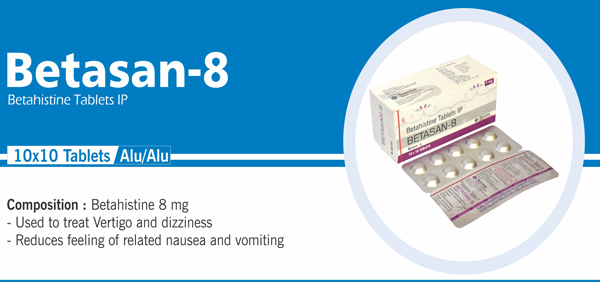
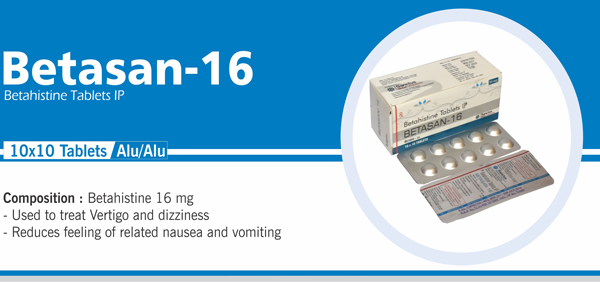
NAUSIA, VOMITING & DIZZINESS
Sr. No. |
Product Name |
Composition |
Remarks |
|---|---|---|---|
103 |
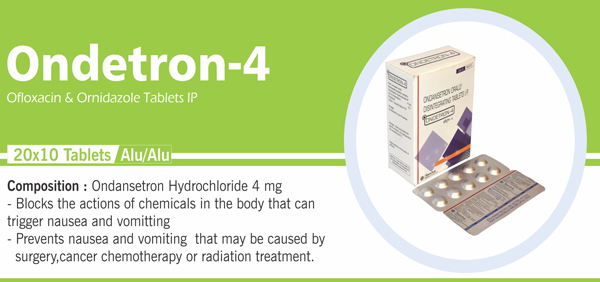
Ondansetron Orally |
Each uncoated orally disintegrating tablet contains: |
|
104 |
Betahistine Tablets IP |
Each uncoated tablet contains: Betahistine Hydrochloride IP 8 mg |
|
105 |
Betahistine Tablets IP |
Each uncoated tablet contains: Betahistine Hydrochloride IP 16 mg |
|
106 |
Cinnarizine Tablets IP 25 mg |
Each uncoated tablet contains : Cinnarizine IP 25 mg |
|
107 |
Cinnarizine and Domperidone |
Each uncoated tablet contains : Cinnarizine IP 20 mg |
|
VITAMINES & DIETRY SUPPLEMENTS DRUGS
Sr. No. |
Product Name |
Composition |
Remarks |
|---|---|---|---|
108 |
Glucosamine, MSM and |
Each film coated tablet contains: Glucosamine Sulphate |
|
109 |
Calcium Carbonate, Vitamin D3, Magnesium Hydroxide and |
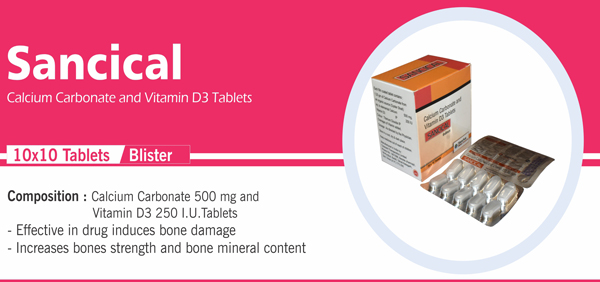
Each Film Coated Tablet Contains : Calcium Carbonate IP 1250 mg |
|
110 |
Citicoline Tablets 500 mg |
Each film coated tablet contains: Citicoline 500 mg |
|
111 |
Methylcobalamin, Alpha Lipoic Acid, Folic Acid, Thiamine and Pyridoxine HCl Capsules |
Each hard gelatin capsule contains: Methylcobalamin JP 1500 mcg Alpha Lipoic Acid USP 100 mg Folic Acid IP 1.5 mg Thiamine Mononitrate IP 10 mg Pyridoxine Hydrochloride IP 3 mg Excipients q.s. Approved colours used in empty hard gelatin capsule shells |
|
112 |
Mecobalamin Tablets 500 mcg |
Each film coated tablet contains |
|
113 |
Mecobalamin Tablets 1500 mcg |
Each film coated tablet contains |
|
114 |
Glucosamine Sulphate & Chondroitin Sulphate Tablets |
Each film coated tablet contains:- Glucosamine Sulphate 500 mg (added as Glucosamine sulphate sodium chloride) |
|
LAXATIVE
Sr. No. |
Product Name |
Composition |
Remarks |
|---|---|---|---|
115 |
Lactulose and Isphaghula |
Each 20 gm of granules contains: Lactulose concentrate USP |
|
ANTI-ALLERGIC
Sr. No. |
Product Name |
Composition |
Remarks |
|---|---|---|---|
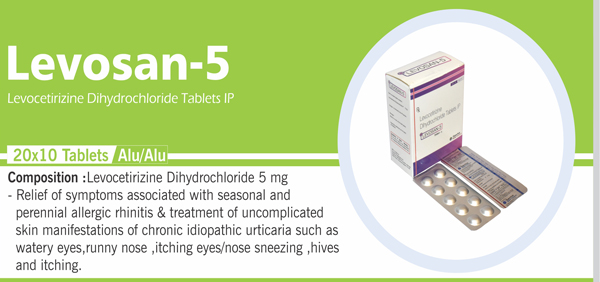
116 |
Levocetirizine Dihydrochloride |
Each uncoated tablet contains: Levocetirizine Dihydrochloride IP 5 mg |
|
OSTEOARTHRITIS DRUGS
Sr. No. |
Product Name |
Composition |
Remarks |
|---|---|---|---|
117 |
Diacerein and Aceclofenac |
Each film coated tablet contains: Diacerein IP 50 mg |
|
118 |
Diacerein and Glucosamine |
Each film coated tablet contains: Diacerein IP 50 mg Glucosamine Sulphate 500 mg Colours : Approved colours |
|
119 |
Glucosamine Tablets USP |
Each film coated tablet contains: Glucosamine Sulphate |
|
RESPIRATORY DRUGS
Sr. No. |
Product Name |
Composition |
Remarks |
|---|---|---|---|
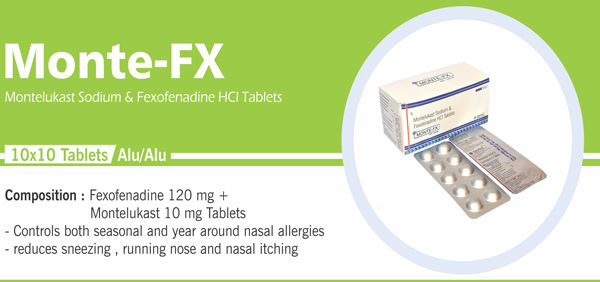
120 |
Montelukast Sodium and |
Each uncoated dispersible tablet contains: Montelukast Sodium IP |
|
121 |
Montelukast Sodium Chewable |
Each uncoated chewable tablet contains: Montelukast Sodium IP |
|
122 |
Montelukast Sodium and |
Each uncoated tablet contains: Montelukast Sodium IP |
|
ANTI-HISTAMINE DRUGS
Sr. No. |
Product Name |
Composition |
Remarks |
|---|---|---|---|
123 |
Fexofenadine |
Each film coated tablet contains |
|
124 |
Fexofenadine |
Each film coated tablet contains |
|
125 |
Montelukast and Fexofenadine |
Each film coated tablet contains: Montelukast Sodium IP |
|
OBESITY DRUGS
Sr. No. |
Product Name |
Composition |
Remarks |
|---|---|---|---|
126 |
Orlistat Capsules USP 60 mg |
Each hard gelatin capsule contains: Orlistat USP 60 mg |
|
127 |
Orlistat Capsules USP 120 mg |
Each hard gelatin capsule contains: Orlistat USP 120 mg |
|
NEUROLOGY AND PSYCHOTROPIC DRUGS
Sr. No. |
Product Name |
Composition |
Remarks |
|---|---|---|---|
128 |
Duloxetine Hydrochloride |
Each enteric coated tablet contains |
|
129 |
Duloxetine Hydrochloride |
Each enteric coated tablet contains |
|
130 |
Duloxetine Hydrochloride |
Each Enteric coated tablet contains |
|
131 |
Clonazepam Tablets IP 0.5 mg |
Each Uncoated tablet contains |
|
132 |
Clonazepam Tablets IP 1 mg |
Each Uncoated tablet contains |
|
133 |
Clonazepam Tablets IP 2 mg |
Each Uncoated tablet contains |
|
134 |
Clonazepam Dispersible Tablets |
Each Dispersible tablet contains |
|
135 |
Disulfiram Tablets IP 250 mg |
Each Uncoated tablet contains Disulfiram IP 250 mg Colour: Approved colours |
|
136 |
Clonazepam mouth dissolving |
Each Mouth dissolving tablet contains |
|
137 |
Clonazepam mouth dissolving |
Each Mouth dissolving tablet contains |
|
138 |
Aripiprazole Tablets IP 10 mg |
Each uncoated tablet contains Aripiprazole IP 10 mg Colour: Approved colours |
|
139 |
Aripiprazole Tablets IP 15 mg |
Each uncoated tablet contains Aripiprazole IP 15 mg Colour: Approved colours |
|
140 |
Aripiprazole Tablets IP 20 mg |
Each uncoated tablet contains Aripiprazole IP 20 mg Colour: Approved colours |
|
141 |
Aripiprazole Tablets IP 30 mg |
Each uncoated tablet contains Aripiprazole IP 30 mg Colour: Approved colours |
|
142 |
Aripiprazole Tablets 5 mg |
Each uncoated tablet contains Aripiprazole IP 5 mg Colour: Approved colours |
|
143 |
Amisulpride Sustained Release |
Each uncoated sustained release tablet contains: |
|
144 |
Amisulpride Sustained Release |
Each uncoated sustained release tablet contains: |
|
145 |
Amisulpride Sustained Release |
Each uncoated sustained release tablet contains: |
|
146 |
Escitalopram oxalate and |
Each film coated tablet contains |
|
147 |
Escitalopram oxalate and |
Each film coated tablet contains |
|
148 |
Piracetam Tablets 400 mg |
Each film coated tablet contains Piracetam IP 400 mg Colour: Erythrosine |
|
149 |
Piracetam Tablets 800 mg |
Each film coated tablet contains Piracetam IP 800 mg Colour: Sunset Yellow FCF |
|
150 |
Escitalopram oxalate Tablets IP |
Each film coated tablet contains |
|
151 |
Escitalopram oxalate Tablets IP |
Each film coated tablet contains |
|
152 |
Escitalopram oxalate Tablets IP |
Each film coated tablets contains Escitalopram oxalate IP Equivalent to Escitalopram 5 mg Colour: Approved colours |
|
153 |
Divalproex sodium Tablets IP |
Each film coated extended release tablet contains |
|
154 |
Divalproex sodium Tablets IP |
Each film coated extended release tablet contains |
|
155 |
Divalproex sodium Tablets IP |
Each film coated extended release tablet contains |
|
156 |
Divalproex sodium Tablets IP |
Each film coated extended release tablet contains |
|
157 |
Divalproex sodium Tablets |
Each film coated extended release tablet contains |
|
158 |
Betahistine hydrochloride |
Each uncoated tablet contains: Betahistine hydrochloride IP 6 mg |
|
159 |
Betahistine hydrochloride |
Each uncoated tablet contains: Betahistine hydrochloride IP 12 mg |
|
160 |
Trihexyphenidyl Hydrochloride |
Each Uncoated tablet contains |
|
161 |
Risperidone Tablets USP 1 mg |
Each film coated tablet contains Risperidone BP 1 mg Colour: Approved colours |
|
162 |
Risperidone Tablets USP 2 mg |
Each film coated tablet contains Risperidone BP 2 mg Colour: Approved colours |
|
163 |
Risperidone Tablets USP 3 mg |
Each film coated tablet contains Risperidone BP 3 mg Colour: Approved colours |
|
164 |
Risperidone Tablets USP 4 mg |
Each film coated tablet contains Risperidone BP 4 mg Colour: Approved colours |
|
165 |
Oxcarbazepine Tablets IP 150 mg |
Each uncoated tablet contains: Oxcarbazepine IP 150 mg Colours: Approved colours |
|
166 |
Oxcarbazepine Tablets IP 300 mg |
Each uncoated tablet contains: Oxcarbazepine IP 300 mg Colours: Approved colours |
|
167 |
Oxcarbazepine Tablets IP 450 mg |
Each uncoated tablet contains: Oxcarbazepine IP 450 mg |
|
168 |
Oxcarbazepine Tablets IP 600 mg |
Each uncoated tablet contains: Oxcarbazepine IP 600 mg |
|
169 |
Phenytoin sodium Tablets IP 50 mg |
Each film coated tablet contains Phenytoin sodium IP 50 mg Colour: Approved colours |
|
170 |
Phenytoin sodium Tablets IP |
Each film coated tablet contains Phenytoin sodium IP 100 mg Colour: Approved colours |
|
171 |
Dothiepin Hydrochloride Tablets |
Each film coated tablet contains Dothiepin Hydrochloride IP 25 mg Colour: Ponceau 4 R |
|
172 |
Dothiepin Hydrochloride Tablets |
Each film coated tablet contains Dothiepin Hydrochloride IP 50 mg Colour: Tartrazine |
|
173 |
Dothiepin Hydrochloride Tablets |
Each film coated tablet contains Dothiepin Hydrochloride IP 75 mg Colour: Ponceau 4 R |
|
174 |
Fluoxetine Hydrochloride |
Each film coated tablet contains |
|
175 |
Fluoxetine Hydrochloride |
Each film coated tablet contains |
|
176 |
Fluoxetine Hydrochloride |
Each film coated tablet contains |
|
177 |
Fluoxetine Hydrochloride |
Each hard gelatin capsule contains: Fluoxetine Hydrochloride IP Equivalent to Fluoxetine 10 mg |
|
178 |
Fluoxetine Hydrochloride |
Each hard gelatin capsule contains |
|
179 |
Haloperidol Tablets IP 0.25 mg |
Each Uncoated tablet contains Haloperidol IP 0.25 mg Colour: Approved colours |
|
180 |
Haloperidol Tablets IP 1.5 mg |
Each Uncoated tablet contains Haloperidol IP 1.5 mg Colour: Approved colours |
|
181 |
Haloperidol Tablets IP 5 mg |
Each Uncoated tablet contains Haloperidol IP 5 mg Colour: Approved colours |
|
182 |
Haloperidol Tablets IP 10 mg |
Each Uncoated tablet contains Haloperidol IP 10 mg Colour: Approved colours |
|
183 |
Haloperidol Tablets IP 20 mg |
Each Uncoated tablet contains |
|
184 |
Lithium carbonate Tablets IP |
Each Uncoated tablet contains |
|
185 |
Lithium carbonate CR Tablets |
Each Film coated controlled released tablet contains |
|
186 |
Levetiracetam Tablets USP |
Each film coated tablet contains: Levetiracetam IP 500 mg Colour: Approved Colours |
|
187 |
Levetiracetam Tablets USP |
Each film coated tablet contains: Levetiracetam IP 750 mg Colour: Approved Colours |
|
188 |
Levetiracetam Tablets USP |
Each film coated tablet contains: Levetiracetam IP 1000 mg Colour: Approved Colours |
|
189 |
Carbamazepine CR Tablets IP |
Each Uncoated controlled release tablet contains |
|
190 |
Flunarizine Dihydrochloride |
Each Uncoated tablet contains |
|
191 |
Flunarizine Dihydrochloride |
Each Uncoated tablet contains |
|
192 |
Mirtazapine Tablets IP 15 mg |
Each film coated tablet contains Mirtazapine IP 15 mg Colour: Iron Oxide Yellow |
|
193 |
Mirtazapine Tablets IP 30 mg |
Each film coated tablet contains Mirtazapine IP 30 mg Colour: Titanium Di Oxide |
|
194 |
Mirtazapine Tablets IP 7.5 mg |
Each film coated tablet contains Mirtazapine IP 7.5 mg Colour: Tartrazine |
|
195 |
Alprazolam Tablets IP 0.25 mg |
Each Uncoated tablet contains Alprazolam IP 0.25 mg Colour: Brilliant Blue FCF & Tartrazine |
|
196 |
Alprazolam Tablets IP 0.5 mg |
Each Uncoated tablet contains Alprazolam IP 0.5 mg Colour: Erythrosine |
|
197 |
Alprazolam Tablets IP 1 mg |
Each Uncoated tablet contains Alprazolam IP 1 mg Colour: Sunset Yellow FCF |
|
198 |
Carbamazepine Tablets IP 100 mg |
Each Uncoated tablet contains |
|
199 |
Carbamazepine Tablets IP 200 mg |
Each Uncoated tablet contains |
|
200 |
Amitriptyline Hydrochloride |
Each film coated tablet contains Amitriptyline Hydrochloride IP10mg Colour: Sunset Yellow FCF |
|
201 |
Amitriptyline Hydrochloride |
Each film coated tablet contains Amitriptyline Hydrochloride IP 25mg Colour: Sunset Yellow FCF & Indigo |
|
202 |
Amitriptyline Hydrochloride |
Each film coated tablet contains Amitriptyline Hydrochloride IP 50 mg Colour: Ponceau 4R |
|
203 |
Olanzapine Tablets IP 2.5 mg |
Each film coated tablet contains Olanzapine IP 2.5 mg Colour: Approved colours |
|
204 |
Olanzapine Tablets IP 5 mg |
Each film coated tablet contains Olanzapine IP 5 mg Colour: Approved colours |
|
205 |
Olanzapine Tablets IP 7.5 mg |
Each film coated tablet contains Olanzapine IP 7.5 mg Colour: Approved colours |
|
206 |
Olanzapine Tablets IP 10 mg |
Each film coated tablet contains Olanzapine IP 10 mg Colour: Approved colours |
|
207 |
Olanzapine Tablets IP 15 mg |
Each film coated tablet contains Olanzapine IP 15 mg Colour: Approved colours |
|
208 |
Olanzapine Tablets IP 20 mg |
Each film coated tablet contains Olanzapine IP 20 mg Colour: Approved colours. |
|
209 |
Lorazepam Tablets BP |
Each Uncoated tablet contains Lorazepam BP 1 mg Colour: Approved colours |
|
210 |
Lorazepam Tablets BP |
Each Uncoated tablet contains Lorazepam BP 2 mg Colour: Approved colours |
|
211 |
Sodium Valproate and Valproic acid Tablets |
Each film coated controlled release tablet contains |
|
212 |
Sodium Valproate and Valproic acid Tablets |
Each film coated controlled release tablet contains |
|
213 |
Propranolol Hydrochloride |
Each Uncoated tablet contains |
|
214 |
Propranolol Hydrochloride |
Each Uncoated tablet contains |
|
215 |
Propranolol Hydrochloride |
Each Uncoated tablet contains |
|
216 |
Quetiapine Fumarate Tablets IP |
Each film coated tablet contains |
|
217 |
Quetiapine Fumarate Tablets IP |
Each film coated tablet contains |
|
218 |
Quetiapine Fumarate Tablets IP |
Each film coated tablet contains |
|
219 |
Quetiapine Fumarate Tablets |
Each film coated tablet contains |
|
220 |
Quetiapine Fumarate Tablets |
Each film coated tablet contains |
|
221 |
Carbamazepine CR Tablets IP |
Each Uncoated controlled release tablet contains |
|
222 |
Carbamazepine CR Tablets IP |
Each Uncoated controlled release tablet contains |
|
223 |
Clozapine Tablets IP 100 mg |
Each uncoated tablet contains: Clozapine IP 100 mg |
|
224 |
Clozapine Tablets IP 25 mg |
Each uncoated tablet contains: Clozapine IP 25 mg |
|
225 |
Clozapine Tablets IP 50 mg |
Each uncoated tablet contains: Clozapine IP 50 mg |
|
226 |
Sertraline Hydrochloride Tablets |
Each film coated tablet contains |
|
227 |
Sertraline Hydrochloride Tablets |
Each film coated tablet contains |
|
228 |
Sertraline Hydrochloride Tablets |
Each film coated tablet contains |
|
229 |
Topiramate Tablets IP |
Each film coated tablet contains Topiramate IP 25 mg Colour: Red Iron Oxide |
|
230 |
Topiramate Tablets IP |
Each film coated tablet contains Topiramate IP 50 mg Colour: Ponceau 4R |
|
231 |
Topiramate Tablets IP |
Each film coated tablet contains Topiramate IP 100 mg Colour: Titanium Di Oxide IP |
|
232 |
Amisulpride Tablets IP 50 mg |
Each uncoated tablet contains |
|
233 |
Amisulpride Tablets IP 100 mg |
Each uncoated tablet contains |
|
234 |
Amisulpride Tablets IP 200 mg |
Each uncoated tablet contains |
|
235 |
Venlafaxine Hydrochloride |
Each Extended release tablet contains |
|
236 |
Venlafaxine Hydrochloride |
Each Extended release tablet contains |
|
237 |
Venlafaxine Hydrochloride |
Each Extended release tablet contains |
|
238 |
Sodium Valproate and Valproic acid Tablets |
Each film coated controlled release tablet contains |
|
239 |
Baclofen Tablets IP 5 mg |
Each uncoated tablet contains: Baclofen IP 5 mg |
|
240 |
Baclofen Tablets IP 25 mg |
Each uncoated tablet contains: Baclofen IP 25 mg |
|
241 |
Baclofen Tablets IP 10 mg |
Each uncoated tablet contains: Baclofen IP 10 mg |
|
NEW LAUNCH PRODUCTS
Sr. No. |
Product Name |
Composition |
Remarks |
|---|---|---|---|
242 |
AMBSAN-SYP |
AMBROXOL 15MG + TERBUTALINE SULPHATE 1.25MG + GUIPHENESIN 50MG + MENTHOL 2.5MG (PER 5 ML) |
|
243 |
B-COMSAN-SYP |
B-COMPLEX + LYSINE 375 MG (PER 10 ML) |
|
244 |
CYPROSAN-SYP |
CYPROHEPTADINE 2MG+ TRICHOLINE 275MG (PER 5 ML) |
|
245 |
DEXSAN-SYP |
DEXTROMETHORPHAN 10MG + CPM 2MG + PHENYLEPHERINE 5MG (PER 5 ML) |
|
246 |
FERTUS-SYP |
FERROUS ASCORBATE 30MG + FOLIC ACID 550MCG (PER 5 ML) |
|
247 |
LYMTUS-SYP |
LYCOPENE 6% 6000MCG + MULTIVITAMIN + MULTIMINERAL |
|
248 |
TERBSAN-SYP |
TERBUTALINE SULPHATE 1.25 MG + BROMHEXINE HYDROCHLORIDE 4 MG + GUIPHENESIN 50MG + MENTHOL 0.5MG (PER 5 ML) |
|
249 |
XYMTUS-SYP |
FUNGAL DIASTASE (1:600) 50 MG + PEPSIN (1:3000) 10 MG (PER 15 ML) (SUGAR FREE) |
|
What is Generic Medicine?
Generic drugs have the same strengths and similar descriptions as the originally marked drugs. Additionally, they are developed by essentially the same serious formulation. In addition, these drugs are fundamentally equivalent to marked drugs. In addition, generic drugs are also produced in similar formulations and in similar raw materials.
In addition, important companies distributing generic drugs are highly respected in the business. These drugs are actually justified, assuming we differentiate them from marked and ethical medicines. Apart from this, the company is also manufacturing the best quality Generic medicines.
Generic Medicine Pharma Franchise Company in India | Sanctus Global
The company offers the best range of Sanctus Global generic drugs. Similarly, Sanctus Global is a company that has the best quality raw material for the details of the goods. Thus, the company is also providing best-in-class support and services in the generic segment. Sanctus Global offers the best range of generic drugs and products. The company is also ensuring that the partners are getting the full variety of generic products. In addition, the company is manufacturing a wide range of generic products. Similarly, the company is offering higher positions and services in this non-specialized pharma industry. This manufacturing company is offering excellent Pharmaceutical Conventional products.
Thereafter, the company is manufacturing first-class quality generic drugs and this makes us the main generic product manufacturing unit. Similarly, the company is ensuring that all generic manufacturing products are safe and potent.
Products offered by the Leading Generic Medicine Franchise Company in India
Each generic product made by us is of excellent quality. Additionally, we ensure that our partners are getting the best range of generic products and generic medicines. Additionally, if you are looking for the best among generic producers, then go for Sanctus Global.
We are giving the best nature of generic products at completely reasonable rates. From now on, Sanctus Global is exceptionally well suited for individuals searching for the best products in the non-specialty sector. Similarly, the company makes excellent arrangements for generic medicines. The range of products is mentioned below:
- Antibiotic products range
- Gastro and PPI Range of Commodities
- Pediatric Range of Products
- Injection and injection range
- ortho range of objects
- Multi-vitamin mineral and antioxidant range
- Derma Variety Products
- NSAID and analgesic products
- Gynae Item Range
- Antihistamine and cough cold product range
Why Sanctus Global is the Best Generic PCD Company?
Sanctus Global is the company that provides the best generic product, unlike different companies. The generic range is popular for several reasons. Apart from this, the company is making immense efforts to create best-in-class generic products. Apart from this, the company is offering the best generic services and products.
Suppose you are looking for the leading generic drug franchise company in India, then Sanctus Global is the best choice for you. Along these lines, choose the best generic products to invest in. Additionally, you should consider these variables when choosing a company to deploy a resource too.
- The company has confirmed with ISO (International companies for standardization).
- Additionally, the company is offering generic products that are endorsed by WHO and DCGI
- Every generic product is manufactured with the conventions of good manufacturing practices.
- Apart from this, the company is incorporating the best quality raw materials for the quality manufacturing of generic medicines.
- Thus, you must go for Sanctus Global for the best quality generic products.
Contact Details
Name – Sanctus Global
Contact Details – 9914562999
Email Id. – SALES@SANCTUSGLOBAL.COM
Address – SCO 56-57, 3rd Floor, Sector-17 D, Chandigarh
Most Searched Terms
- generic medicine franchise
- generic medicine franchise company list
- generic medicine company franchise
- generic medicine franchise india
- pcd pharma franchise generic medicine
- franchise for generic medicine
- generic medicine franchise company
- generic pharma companies
- generic pharma companies in india
- top generic pharma companies in india
- pcd generic pharma company
- generic pharma companies franchise
- generic pharma company franchise